PainRelief.com Interview with:
Rajesh Khanna, PhD
Professor and Vice Chair of Research, Department of Pharmacology,
Associate Director of Research, Comprehensive Pain and Addiction Center
University of Arizona

Starting January 2022:
Professor, Department of Molecular Pathobiology
Director, NYU Pain Center
College of Dentistry New York University
PainRelief.com: What is the background for this study?
Response: Chronic pain conditions cause an immense burden on society due to their astonishingly high prevalence and lack of effective treatments. The National Institutes of Health estimates that nearly 100 million people in the United States suffer from chronic pain. Nearly 20-30% of patients prescribed opioids for chronic pain misuse them, according to the National Institute on Drug Abuse. In 2019, nearly 50,000 people in the U.S. died from opioid-involved overdoses and that number increased to nearly 70,000 in 2020. There is clearly an urgent need for non-addictive treatments for chronic pain.
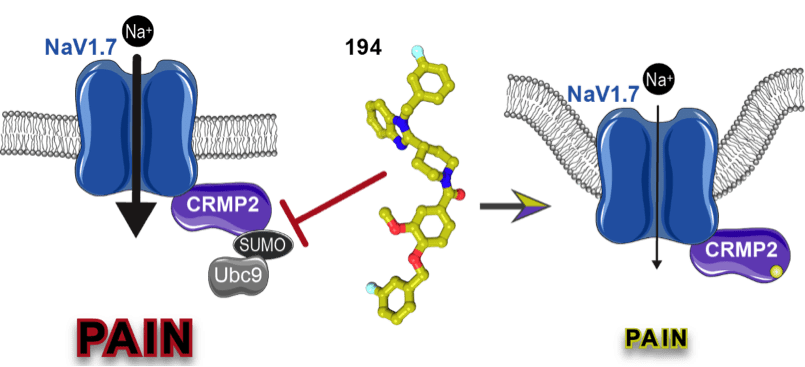
The voltage-gated sodium channel NaV1.7 is preferentially expressed in the peripheral nervous system within ganglia associated with nociceptive pain. This channel modulates the threshold required to fire action potentials in response to stimuli and has been established as a key contributor to chronic pain. Chronic pain states can result from upregulated NaV1.7 expression which has been shown to occur in association with diabetic neuropathy, inflammation, sciatic nerve compression, lumbar disc herniation, and after spared nerve injury. The exact pathways leading to the dysregulation of NaV1.7 are poorly understood, but likely involve mechanisms related to its surface trafficking and regulation via protein-protein interactions.
Our previous work identified the collapsin response mediator protein 2 (CRMP2) as a novel regulator of NaV1.7 function and uncovered the logical coding of CRMP2’s regulatory functions. We found that if CRMP2 is phosphorylated by cyclin dependent kinase 5 at serine 522 and also modified by SUMOylation at lysine 374 by the SUMO conjugating enzyme Ubc9, then NaV1.7 is functional. When not SUMOylated, CRMP2 recruits the endocytic proteins Numb, Nedd4-2, and Eps15, triggering clathrin mediated endocytosis and internalization of NaV1.7. When not at the cell-surface, sodium currents are reduced, alleviating NaV1.7-associated chronic pain. This action of CRMP2 is highly selective for NaV1.7, as no effects on other voltage-gated sodium channel subtypes are observed.
Previous efforts to target NaV1.7 for pain relief have focused on development of direct channel blockers, but this approach has been unsuccessful. Disclosed reasons for failure of these NaV1.7-targeting drugs include issues with:
(a) central nervous system penetration,
(b) lack of selectivity (e.g., of Biogen’s Vixotrigine),
(c) inadequacy of pain models, and
(d) insufficient channel blockade.
These factors culminate in continued action potential firing and failure to relieve pain, which has led to skepticism regarding targeting of NaV1.7.
We hypothesized that targeting CRMP2 with a small molecule to prevent it’s SUMOylation would be a novel and effective approach to indirectly regulating NaV1.7 for the treatment of chronic neuropathic pain.
PainRelief.com: What are the main findings?
Response: To test this hypothesis, we used the known structure of CRMP2 to conduct an in-silico screen of small molecules targeting the SUMOylation site. Subsequent in vitro screening identified compounds capable of preventing modification of CRMP2 with SUMO. We then developed an improved compound, which we call 194, that was effective at relieving pain in preclinical models. In this study, we show that our compound specifically blocks the interaction of Ubc9 with CRMP2 and inhibits its SUMOylation. This results in decreased NaV1.7 currents in sensory neurons, reducing spinal nociceptive neurotransmission.
Our preclinical studies reveal that oral administration of 194 decreases mechanical allodynia in six models of acute and chronic neuropathic pain. Excitingly, we demonstrate that 194 synergizes with commonly used painkillers morphine and gabapentin to relieve pain at subtherapeutic doses. We also found that 194 activates NaV1.7-dependent endogenous opioid signaling. Importantly, our studies showed that our compound has no motor or depressive-like side effects, nor does it exhibit addictive properties.
PainRelief.com: What should readers take away from your report?
Response: We have identified a new compound that alleviates chronic pain in preclinical models using a unique approach to indirectly regulate a voltage-gated sodium channel. This holds promise for development of a new therapy that targets this important pathway without affecting normal pain sensations. Further, our compound has additional potential of co-administration with low doses of opioids to treat pain with commonly used drugs currently on the market with reduced addiction liability.
PainRelief.com: What recommendations do you have for future research as a result of this work?
Response: NaV1.7 emerged as a pain target over 20 years ago but no drugs targeting this channel have made it to the clinical doorstep. Recent studies have revealed previously unappreciated roles of this channel in pain signaling, including the idea that block of this channel must be sufficient to drive an increase in endogenous opioid signaling. Thus, conventional strategies to targeting this channel are likely to fail. New, outside the box approaches, such as the one espoused here, are likely to advance this channel as a bona fide target to treat chronic pain.
This work was supported by grants NS098772, NS120663, DA042852, T32DA024628, CA200417, and CA210857 from the National Institutes of Health. Additional funding to advance the technology was provided by Tech Launch Arizona.
Any disclosures? Several co-authors are cofounders of Regulonix LLC and ElutheriaTx Inc., companies devoted to developing nonopioid drugs and gene therapy approaches for treating chronic pain. Regulonix LLC holds patents for several non-narcotic compounds targeting sodium channels for chronic pain.
Citation:
Selective targeting of NaV1.7 via inhibition of the CRMP2-Ubc9 interaction reduces pain in rodents
SCIENCE TRANSLATIONAL MEDICINE10 Nov 2021Vol 13, Issue 619
DOI: 10.1126/scitranslmed.abh1314
https://www.science.org/doi/10.1126/scitranslmed.abh1314
The information on PainRelief.com is provided for educational purposes only, and is in no way intended to diagnose, cure, or treat any medical or other condition. Always seek the advice of your physician or other qualified health and ask your doctor any questions you may have regarding a medical condition. In addition to all other limitations and disclaimers in this agreement, service provider and its third party providers disclaim any liability or loss in connection with the content provided on this website.
Last Updated on November 17, 2021 by PainRelief.com
